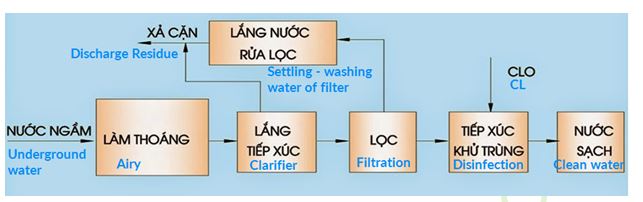
PROCESS OF WATER SUPPLY TREATMENT FOR LIVING ACTIVITIES AT WATER PLANT
1. Reservoir, and preliminary settling:
It creates favourable conditions for the self-cleaning process: settling suspended sediments, reducing the amount of germs due to environmental conditions, performing oxidation reactions due to the effects of dissolved oxygen in water and regulating the circulation amount of flow from the source to the consumption flow supplied by the raw water pumping station to the water treatment plant.
2. Grates and trash screens:
It eliminates floating objects in the water line to protect equipment and improve the cleaning efficiency of treatment facilities. Floating objects and suspended objects in water can be as small as a toothpick when passing through the pump into treatment facilities that may be scattered or rotted to increase the colour and sediment content of the water.
3. Grit chamber:
In surface water sources with large turbidity, (> 250 mg / L) after trash screening, inorganic suspended particles with small size, and greater density than water, hard, capable of fast settling are retained in Grit chamber.
Good condition is created to settle particles with a size greater than or equal to 0.2 mm and a density greater than or equal to 2.6mm, to eliminate the erosion of physical movement and reduce the amount of heavy sediment in flocculation and sedimentation basin.
4. Chemical treating of source water
To limit the growth of algae and water microorganisms, eliminate colour, smell, taste caused by dead microorganisms. Commonly used chemicals are CuSO4, dosage of 0.12 ÷ 0.3 mg / l. The dosage and interval between treatments depends on the composition of raw water as well as the concentration of microorganisms and algae, temperature, alkalinity and CO2 content.
5. Aeration:
Dissolve oxygen from the air into water to oxidize iron II, manganese II to iron III, manganese IV to form hydroxide compounds Fe (OH) 3, Mn (OH) 4 precipitates which are easy to deposit to remove from water by sedimentation and filtration.
Removal of CO2, H2S in water, increasing the pH of water, creating favourable conditions and accelerating oxidation, hydrolysis of iron and manganese, improving the productivity of sedimentation and filtration works in the treatment process for iron and manganese.
Clearing process increases the amount of dissolved oxygen in water, raising the electrode potential of water to facilitate the oxidation of organic substances in the deodorization and discoloration process of water.
There are two methods of ventilation: water is brought into the air and air is brought into the water (mostly water is brought into the air).
The effectiveness of the ventilation process depends on:
- Difference in concentration of gas to be exchanged in 2 phases of gas and water.
- The contact area between the two gas and water phases, the larger the contact area, the faster the gas exchange will take place.
- The contact time between gas and water phases in a building, the greater the contact time, the more thorough the level of exchange.
- Temperature of the environment: an increase in temperature will be beneficial for the process of degassing out of the water and detrimental to the absorption and dissolution of gas into the water.
- The nature of the gas exchanged
6. Preliminary chlorination:
Adding chlorine and water in front of Sedimentation basin and Clarifier
Extending contact time for sterilization when water sources are heavily contaminated
Oxidation of soluble iron in the form of organic compounds, oxidizing dissolved manganese to form the corresponding precipitate.
Oxidizing organic substances to remove colour
Neutralizing ammonia to chloramine with long lasting sterilizing properties
Preventing the growth of algae in the reaction basins and settling basins, destroying the cells of microorganisms producing mucus on the surface of the, increasing the filter cycle time.
Disadvantage:
- Consumption of chlorine is 3 to 5 times higher than the amount of chlorine used to disinfect water behind the Clarifier, increasing the cost of water for treatment.
- The reaction of chlorine with water-soluble substances creates a trihalomotheme compound which is a carcinogen for water users, so it should not be applied to surface water sources containing many organic substances.
7. Chemical mixing:
Facilitate rapid and even dispersion of chemicals into the entire volume of water to be treated. The process of mixing alum requires rapid and even mixing of alum into the water to be treated, because the reaction of hydrolysis to create flocculation is very fast (usually less than 1/10s). If it is not mixed well and long enough, coagulation cores will not be sufficient, firm and even in water; moreover, the sedimentation effect will be poor and alum consuming, other chemicals require mixing thoroughly, and the mixing time is less strict than alum.
8. Coagulation and flocculation reaction:
To create agents capable of binding water-soluble contaminants in suspended form into sediments with the ability to settle in settling basins and to cohesion on the particle surface of the filter media layer at a fast and economical rate.
When neutralized, this positive colloidal system is the nucleus capable of binding to the negative colloids dispersed in water and bonding together to form residues, thus the process of creating an adhesive nucleus called the process of coagulation, the process of bonding dregs and multiplying the flocculation is called the process of flocculation formation.
Alum and iron alum are often used.
9. Sedimentation:
The process of reducing the concentration of suspended sediments in source water by the following measures:
- Deposition of gravity in the sedimentation basins, then sediment particles with a density greater than water in the appropriate hydraulic regime will settle.
- By centrifugal force acting on sediment, in centrifugal sedimentation basins and hydraulic cyclones
- By floating force due to air bubbles sticking to sediment particles in the flotation basins, along with sedimentation, the process of reducing 90 ÷ 95 bacteria in water due to adsorbing and sticking floc particles during sedimentation.
10. Filtering:
A process that not only retains suspended particles in water that are larger than the size of the pores created between the filter particles but also retains iron, organic colloidal particles that cause turbidity and colour, with dimensions smaller than the size of the pores but capable of sticking and absorbing on the surface of the filter layer.
Factors affecting the process of water filtration through particle clarifier basins:
- Filter particle size and particle size distribution in the filter media
- Size, shape, specific gravity concentration and cohesion ability of suspended sediment in the treated water
- Filter speed, filter height, composition of the media and the pressure difference for the loss of a filtration cycle
- Temperature and viscosity of water
11. Absorbing odours, colouring:
Activated carbon powder particles have a very large active surface, capable of adsorbing gas molecules, and molecules of liquid substances dissolved in water that make water taste and colour, onto the surface of this charcoal is out of the water. The water is deodorized and discoloured.
To remove the taste and discoloration of water with activated carbon, two methods can be used:
Bring the treated water according to traditional technology to filter directly through activated carbon clarifier basin
Mix powdered activated carbon to a size of several tens of micrometres into the mixing basin of source water with alum at a dosage of 3 ÷ 15 mg / l to absorb organic substances that cause the taste and colour of water. This method increases the efficiency of flocculation, settling, filtering and sedimentation in settling basins are easier to be treated.
12. Fluorination of water:
After the water treatment process, the amount of fluorine in the water is lower than the standard, so it is necessary to add fluorine to the water.
13. Sterilization:
To ensure bacteriological safety, water before being supplied to consumers must be disinfected.
- Sterilization measures:
- Boiling water
- Using ultraviolet rays
- Using chemicals with high sterilizing effect: ozone, chlorine, etc.
14. Stabilizing water:
It is the process of reducing the aggressiveness of water and implanting on the inner wall of a tube a protective film to insulate water from direct contact with the pipe-making material.
Effect:
- It create rust protection for steel pipes and pipe fittings
- It does not allow water to dissolve lime in the cement component of the inner layer of cast iron pipe and ductile iron pipe, the inner surface of concrete pipes.
Chemicals used to stabilize water are: hexametha phosphate, sodium silicate, soda, lime




